What Are Digital Therapeutics? Definitions and Terminology Explained
Is there a difference between Digital Therapeutics and Digital Medicines? What are around-the-pill DTx? If you are lost in the emerging lingo of the Digital Therapeutics industry, this post is for you

- Digital therapeutics (DTx) are software programs with evidence-based therapeutic capabilities rooted in behavior change
- While digital medicines and digital therapeutics are similar concepts, industry associations are trying to establish a clear terminology
- While two major types of digital therapeutics are emerging, the industry is using words like 'monotherapy' or 'stand-alone DTx' largely interchangeably
What are Digital Therapeutics?
Although the precise definition of a digital therapeutic is still up for some debate, the majority of variations can be summed up with:
Digital therapeutics (DTx) are software solutions that have evidence-based therapeutic capabilities. Like digital therapy or digital medicine, digital therapeutics have a measurable impact on health outcomes.
Digital therapeutics achieve their impact through digital interventions, which typically induce or facilitate specific patient behavior. Examples include increasing medication adherence, enacting self-care instructions, or guiding towards a healthier lifestyle.
What are Digital Medicines? And How Do They Differ from Digital Therapeutics?
The meaning of digital therapeutics and the meaning of digital medicines is often considered to be the same. Still, if you are looking for a concise definition of digital therapeutics and related concepts, you can draw on a proposed classification from the Digital Medicines Society (DiMe), The Digital Therapeutics Alliance and some others. For them, the difference derives from the availability of real-world evidence:
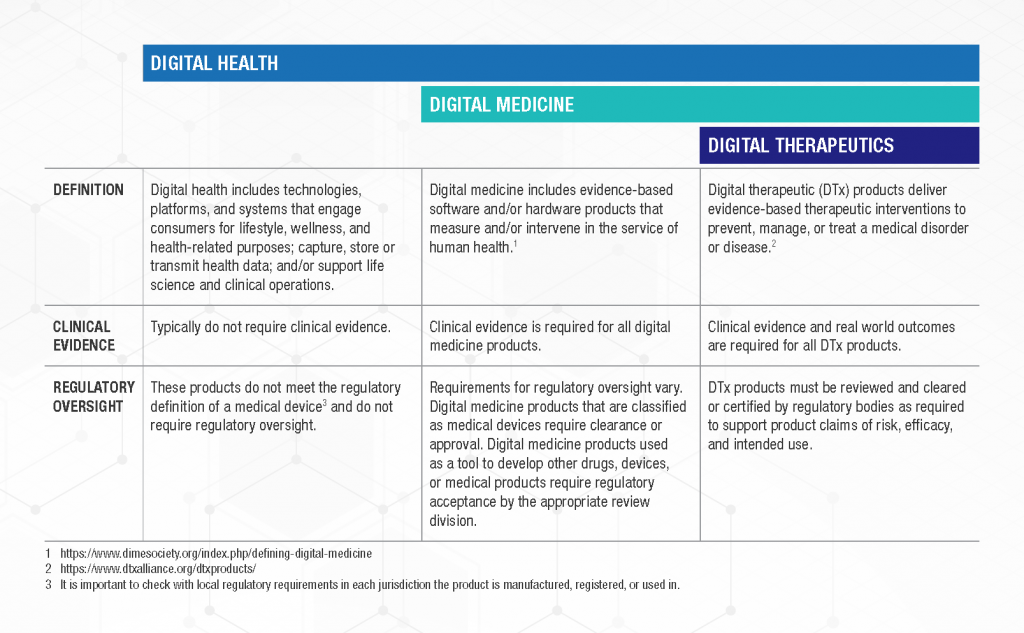
Time will tell if this classification of digital health persists. In the meantime, whilst you are reading or discussing, you can assume that others are likely to use these terms broadly and rather interchangeably.
Prescription Digital Therapeutics vs ‘Around-the-Pill’
As the world of digital therapeutics has developed, two distinct forms of DTx have emerged: ‘prescription’ or ‘standalone’ digital therapeutics and ‘around-the-pill’ digital therapeutics.
What are Prescription DTx or Monotherapy Digital Therapeutics?
‘Prescription’ or ‘standalone’ digital therapeutics are those designed to work independently of regular pharmaceuticals and are usually designed to prevent or treat health conditions. Should a patient be diagnosed with prediabetes, for instance, an effective standalone DTx can successfully enact lifestyle changes that can prevent the condition from progressing to type 2 diabetes. Drawing on lingo from the world of pharmaceuticals, prescription DTx are also referred to as 'monotherapy digital therapeutics'.
What are Around-the-Pill DTx or Combination Digital Therapeutics?
'Around-the-pill’ digital therapeutics have evidence for delivering their impact in combination with another treatment, typically a drug. They deliver their results in ways such as by helping patients taking the right dose at the right time or better managing symptoms and side effects of their disease or treatment.
What Is the Definition of a Digital Therapeutics Company?
Although in principle anyone can develop digital therapeutics, it takes a set of highly specific capabilities to do so well. Enacting behavior change – and making those changes stick – is a task that is rooted in unraveling decades worth of research and behavior change theories and repurposing them for the digital era. It also requires engaging with patients to gain a true understanding of their needs.
Furthermore, designers and developers must deliver the behavior change elements in a usable and engaging package, along with additional functionalities that help support patients. Finally, the regulatory strategy needs to be sound from the start.
What is 'Digital Therapeutics Regulation'?
In simple terms, software that is therapeutic in nature is classified as a medical device ('SaMD' - Software as Medical Device). While the exact rules depend on the regulatory environment (e.g. FDA for the US, MDR for Europe), SaMD typically requires rigid management of quality and risk during development and operation. Beyond medical device regulation, digital therapeutics in many countries fall under specific regulations concerning privacy, handling of health-related data, and cybersecurity.
Are Platforms the Future of Digital Therapeutics?
Whether standalone or around-the-pill, many digital therapeutics share fundamental requirements such as proven user engagement. As digital therapeutics become more widely accepted and regulated, the scope and scale of their use are only likely to increase.
Bearing the above in mind, it is not desirable or necessary for each solution to be developed from the ground up, go through dozens of iterative refinements, be clinically tested, and achieve regulatory approval. Rather, developing tailored and targeted components that operate on a platform that provides the core features is a more efficient and cost-effective approach for a variety of DTx.
This is the approach we are taking with MyTherapy, effectively making it an ‘Operating System for Digital Therapeutics’, onto which specially developed app modules and programs can be installed.
For us, it means improvements to the MyTherapy platform benefits all of our partner products while allowing us to devote more time to individually tailored components. For our partners, it means getting products to market quicker, with less risk, and at a lower cost, without sacrificing quality.
If you want to hear more about our vision for the future of digital therapeutics and discuss partnership opportunities, don’t hesitate to get in touch.